We conducted a survey of research projects and organizations related to dementia that make use of the PPP model. The survey also included representative organizations that are making progress in research on the formation of PPPs.
■Dementia research programs around the world-Large-scale, multi-institutional joint studies and partnerships
| Abbreviation | WW-ADNI | DIAN | A4 study |
|---|---|---|---|
| Official Name | World Wide Alzheimer's Disease Neuroimaging Initiative | Dominantly Inherited Alzheimer Network | Anti-Amyloid Treatment in Asymptomatic Alzheimer’s study |
| Year of Establishment | 2003 | 2008 | 2014 |
| Management | Alzheimer's Association | Washington University School of Medicine | Alzheimer's Therapeutic Research Institute |
| Collaborated organizations | 25 companies, 150 academic organizations, Foundation for the National Institutes of Health The National Institute on Aging | The National Institute on Aging | The National Institute on Ageing, Eli Lilly and Company, Philanthropic institutions |
| Aims | To help define the rate of progression of mild cognitive impairment and Alzheimer’s disease To develop improved methods for identifying the appropriate patient populations to participate in clinical trials. To standardize the methods used for conducting imaging scans and gathering and testing fluid samples | To find solutions to treat or To find solutions to treat or prevent Dominantly Inherited Alzheimer’s Disease (A rare form of Alzheimer’s), and potentially, all forms of Alzheimer’sprevent Dominantly Inherited Alzheimer’s Disease (A rare form of Alzheimer’s), and potentially, all forms of Alzheimer’s | To test whether a new investigational treatment, called solanezumab, an anti-amyloid antibody, can slow memory loss caused by Alzheimer’s disease |
■Dementia research programs around the world-Large-scale study-funding organizations
| Abbreviation | IMI | EPAD | DPUK | GAP | AMP | IADRP | GAAIN |
|---|---|---|---|---|---|---|---|
| Official Name | Innovative Medicines Initiative | European Prevention of Alzheimer Dementia Consortium | The MRC Dementias Platform UK | Global Alzheimer's Platform | Accelerating Medicine Partnership | International Alzheimer's and Related Dementias Research Portfolio | The Global Alzheimer’s Association Interactive Network |
| Year of Establishment | 2008 | 2015 | 2014 | 2013 | 2014 | 2010 | 2010 |
| Management | European Union、European Federation for Pharmaceutical Industries and Associations(EFPIA) | Dr. Serge Van der Geyten | Medical Research Council | The GAP Foundation | Foundation for the National Institutes of Health(FNIH) | NIH, National Institute on Aging and the Alzheimer’s Association | USC Laboratory of Neuro Imaging, USC Mark and Mary Stevens Neuroimaging and Informatics institute, Keck School of Medicine of USC |
| Collaborated organizations | Janssen Pharmaceutical, Eisai Pharma, Bayer Pharma, etc | 35 organisations including Eurpean Union, EFPIA companies and Universities, and research organisations | The National Institute on Aging (NIA), the National Institute of Bioimaging and Bioengineering (NIBIB), Araclon Biotech, Cambridge Cognition, MedImmune, the global biologics research & development arm of AstraZeneca, GlaxoSmithKline, Invicro, Ixico, Janssen Pharmaceuticals, in collaboration with Johnson & Johnson Innovation, SomaLogic. | Janssen, Eli Lilly and Company, Roche, Lundbeck | Food and Drug dministration(FDA), National Institutes of Health (NIH), AbbVie, Biogen, Bristol-Myers Squibb, GlaxoSmithKline, Johnson & Johnson, Eli Lily and Company, Merck, Pfizer, Sanofi, Takeda Pharmaceutical Company, Alzheimer‘s Association, Alzheimer’s Drug Discovery, American Diabetes Association, etc. | 40 organizations from 10 countries including, Administration on Ageing, CDC, Alzheimer’s Association, BrightFocus Foundation, Canadian Institute of Health Research, Alzheimer’s Research UK | 24 organizations including, Alzheimer’s Association, DIAN, Aibl, ADNI, I-ADNI, CAMD |
| Aims | To develop next generation vaccine, medicine, treatments | To improve the understanding of the early stages of Alzheimer’s disease and deliver new preventative treatments | To help define the rate of progression of mild cognitive impairment and Alzheimer’s disease To develop improved methods for identifying the appropriate patient populations to participate in clinical trials. To standardize the methods used for conducting imaging scans and gathering and testing fluid samples | To speed up the delivery of innovative therapies to those afflicted with Alzheimer’s by reducing the time and cost of Alzheimer’s disease (AD) clinical trials. | To transform the current model for developing new diagnostics and treatments by jointly identifying and validating promising biological targets for therapeutics | Enable public and private funders of Alzheimer’s research to coordinate research planning, leverage resources, avoid duplication of funding efforts and identify new opportunities in promising areas of growth. | Promotes big data sharing among a federated, global network of data partners who are studying Alzheimer’s disease and other dementias. To address the need to coordinate and leverage existing resources to advance research into the root causes of the disease, improve diagnostics and discover novel therapeutics, and find better ways to deliver care. |
■Dementia research programs in Japan – Large-scale, multi-institutional joint studies, partnerships, and large-scale study-funding organizations
| Abbreviation | J-ADNI | IROOP | ORANGE Registry |
|---|---|---|---|
| Official Name | Japanese-Alzheimer’s Disease Neuroimaging Initiative | Integrated Registry Of Orange Plan | Organized Registration for the Assessment of dementia on Nation-wide General consortium toward Effective treatment in Japan |
| Year of Establishment | 2007 | 2016 | 2015 |
| Management | University of Tokyo | National Center of Neurology and Psychiatry (NCNP), Japan Agency for Medical Research and Development | National Center for Geriatrics and Gerontology, National Center of Neurology and Psychiatry (NCNP), Tokyo dementia care research and training center, Ministry of Health, Labor and Welfare |
| Collaborated organizations | 30 academic organizations | National Center for Geriatrics and Gerontology, Yokohama Brain and Spine Center, Osaka City University Faculty of Medicine | 30 academic organizations |
| Aims | To conduct joint clinical studies with other facilities to develop criteria for evaluating the efficacy of AD drugs. To track healthy elderly patients, MCI patients, and early AD patients for 2 to 3 years. | To clarify symptoms that appear before the onset of dementia and to elucidate risk factors by improving lifestyles, habits, etc. To promote clinical research and clinical trials for the development of drugs expected to improve cognitive function. | To establish an appropriate care method for dementia by collecting information on people in various stages of health including: Healthy, Preclinical stage, MCI, Mild dementia, Moderate dementia, and Advanced dementia. |
This research identified that the essential element of an effective PPP is clarity in organizational values, mission, and goals.
Furthermore, those interviewed commented on the strong need for Japan to be involved in international research collaborations including those using the PPP model. To achieve this, it is important to strengthen the basic foundations of clinical trial research in Japan, including the practices by which participants and workers who can collaborate internationally are recruited. It is also crucial to secure stable funding streams to make all of this possible.
In a study of fields that can be expected to be promoted by a PPP excluding drug discovery, we conducted interviews and other surveys with care service providers and related services, local governments, town planning experts, robotics experts, and IoT researchers. We also conducted a survey of precedent cases of research promoted by PPPs on topics other than dementia and used them as references for modeling the construction of a dementia PPP.
■Challenges facing stakeholders undertaking advanced efforts in areas related to dementia and their needs
■Suggestions for the PPP mechanism in Japan
Interviews identified the need for a PPP to facilitate knowledge sharing within the research community and to assess new technology. Also, interviews highlighted the importance of having a clear shared vision and mission within the PPP and of promoting transparency and impartiality in project management.
Interviews with stakeholders revealed that they are highly interested in the role that Japan is expected to fill in the WDC and in other global policy planning endeavors. After sharing opinions with the WDC on this matter, the WDC decided to investigate the current situation of and future topics in each field it focuses on.
■Suggestions and expectations for Japan from the WDC
These conversations allowed for opinions on the concept of the PPP model to be exchanged and they reaffirmed the need for Japan to actively contribute to the WDC. They also stressed the importance of long-term strategic efforts for effective drug discovery and the standardization of care and evaluation methods.
A multinational comparative analysis was carried out to examine the current status of international PPPs for dementia research. It identified elements and functions required for cooperation between industry, government, and academia regarding dementia research in Japan.

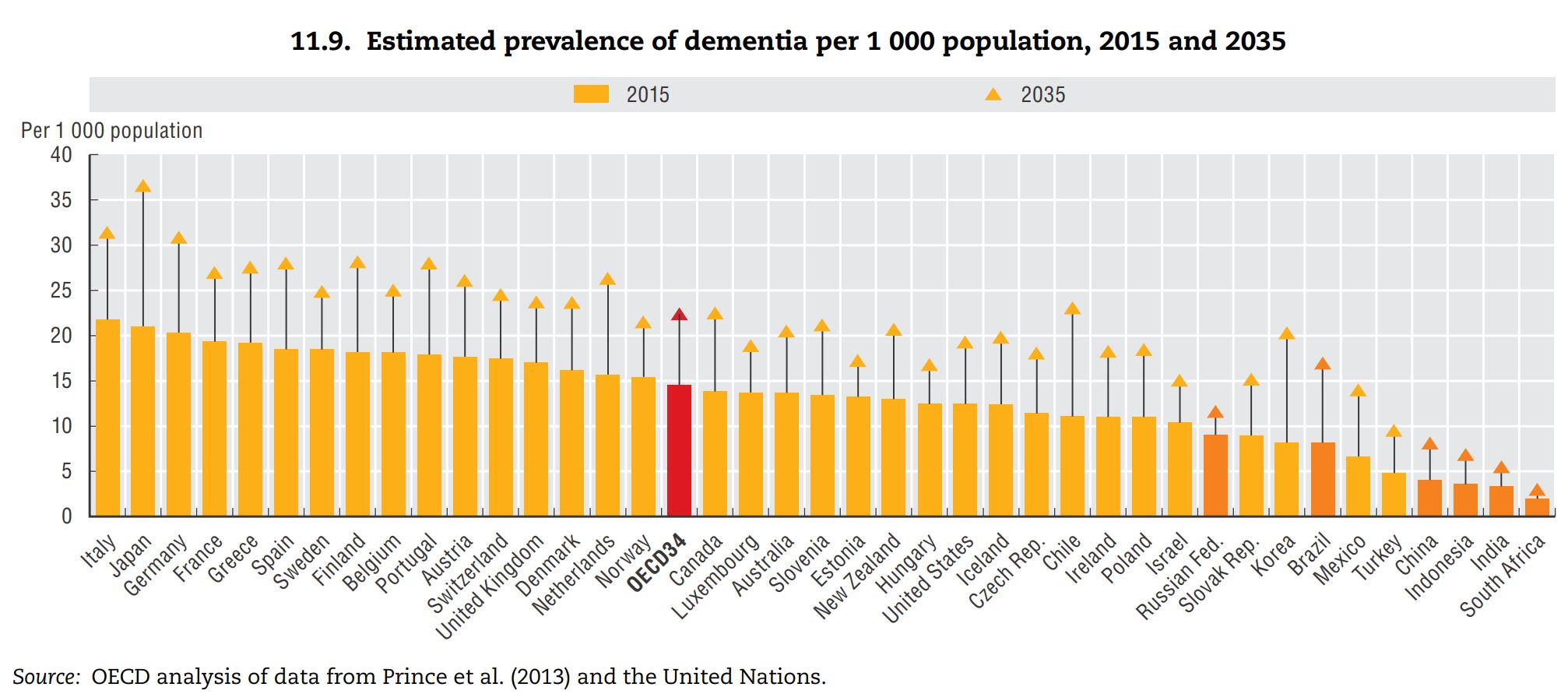
Source: OECD Health Policy Studies Addressing Dementia
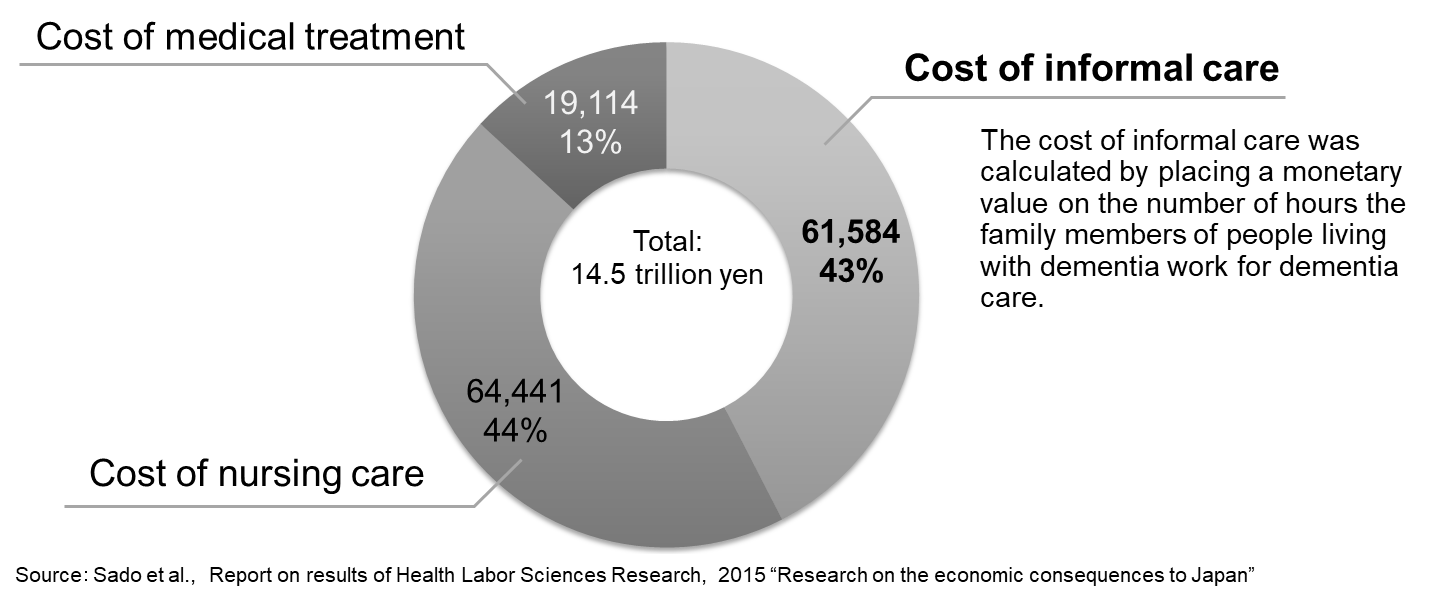
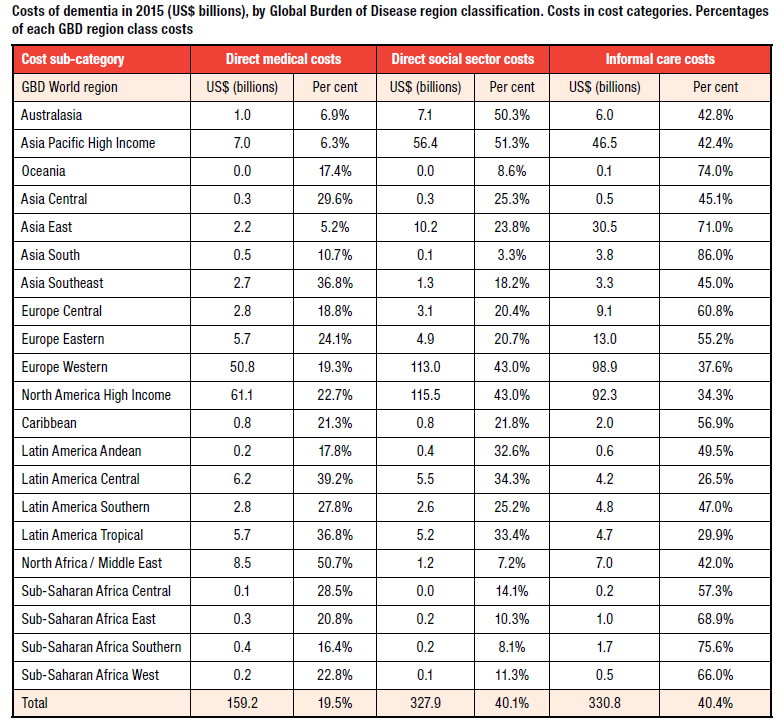
Source: World Alzheimer Report 2015
■Dementia-related national strategies and guidelines in Western countries and Japan
| United States | United Kingdom | Japan | ||
|---|---|---|---|---|
| Dementia related national strategies | The National Alzheimer’s Project Act (NAPA) | Living well with dementia: A National Dementia Strategy | Comprehensive Strategy to Accelerate Dementia Measures (New Orange Plan) | |
| Guidance/ Guidelines | Name | Guidance for Industry, Alzheimer’s Disease : Developing Drugs for the Treatment of Early Stage Disease | Draft guideline on the clinical investigation of medicines for the treatment of Alzheimer’s disease and other dementias | Guideline on the clinical evaluation of medicines for the treatment of Alzheimer’s Disease (AD) |
| Explanation | The FDA’s current approach covers areas such as: diagnostic methods for early-stage Alzheimer’s disease patients who are participating in clinical trials; patient selection and diagnostic methods for patients for whom the disease has the possibility of progressing; directions for setting the endpoints of clinical trials; and directions for using biomarkers to measure treatment outcomes. | These guidelines aim to create new diagnostic criteria for AD, including early-stage and asymptomatic AD patients based on the results of impact assessments; select treatments based on outcomes for each stage of progress of AD; enable the effective use of biomarkers; and design trials to determine the long - term effectiveness and safety of dementia treatment drugs. | Currently being produced. | |
The results of this project emphasized the need for Japan to become involved in improving relevant guidance and guidelines for dementia. It also emphasized the importance of developing a registry framework for clinical trials in order to further advance global collaborations in dementia research.
An advisory board and an expert committee that brought together people with dementia and their families and representatives from industry, government, and academia was established in order to exchange opinions regarding the promotion of research by a PPP. (*All affiliations are current at the time of writing)

Three key approaches that will enable Japan to demonstrate leadership in Asia as a society experiencing advanced aging have been identified. First, the PPP must provide a platform for data collection and assessment that can facilitate discussion among organizations from various sectors. Second, the PPP must also function as a high-quality dementia registry that can enable Japan’s participation in large-scale international clinical trials. Last but not least, the PPP should function as an organization that can raise awareness of dementia among the public and thereby promote understanding and acceptance.